Brazil’s health regulator has granted emergency approval to two COVID-19 vaccines as the country gears up for a mass inoculation campaign during a devastating second wave of the pandemic.
The Anvisa regulator on Sunday approved vaccines from China’s Sinovac Biotech and Britain’s AstraZeneca for emergency use in Brazil, which has recorded more than 209,000 deaths linked to the novel coronavirus since the start of the crisis.
Anvisa’s board of directors voted unanimously to approve both vaccines after almost five hours of deliberations.
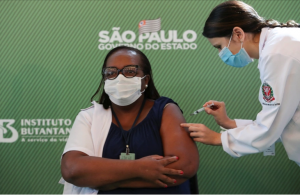
Minutes after the vote, Monica Calazans, a 54-year-old nurse in Sao Paulo, became the first person to be inoculated in Brazil, receiving the Chinese vaccine known as CoronaVac.
Brazilian President Jair Bolsonaro, a coronavirus skeptic who has refused to take a vaccine himself, is under pressure to start inoculations as the country’s death and case counts continue to mount rapidly.
Brazil has recorded more than 8.45 million cases of COVID-19 to date, according to Johns Hopkins University, the third-highest tally in the world after the United States and India.
Delays with vaccine shipments and testing results have held up vaccinations in Brazil so far, however.
Bolsonaro’s government was planning to kick off a national immunization program this week, but it is still waiting on shipments of the AstraZeneca vaccine.
Sao Paulo Governor Joao Doria, who oversees the Butantan biomedical center that partnered with Sinovac in Brazil, said on Sunday that widespread vaccinations could start immediately.
But Health Minister Eduardo Pazuello told a news conference that the government would start distributing the vaccines to states on Monday morning. Brazil could eventually vaccinate one million people per day, he said.
(Source: Al-Jazeera)